Deducing the Allowed Quantum Numbers of an Atomic Electron
To completely describe an electron in an atom four quantum numbers are needed. O ELECTRONIC STRUCTURE Deducing the allowed quantum numbers of an atomic electron List all possible values of the magnetic quantum number m for a 3d electron.
Answered What Is The Smallest Possible Value Of Bartleby
What Is The Smallest Possible Value Of The Principal Quantum Number N Of The State.
. 0 1 2 3 and so on. View Deducing the allowed quantum numbers of an atomic electronpng from CHEM 121 at University of New Mexico. Deducing the allowed quantum numbers of an atomic electron Problem.
Chemistry questions and answers. N X This problem has been solved. Show transcribed image text O ELECTRONIC STRUCTURE Deducing the allowed quantum numbers of an atomic ele.
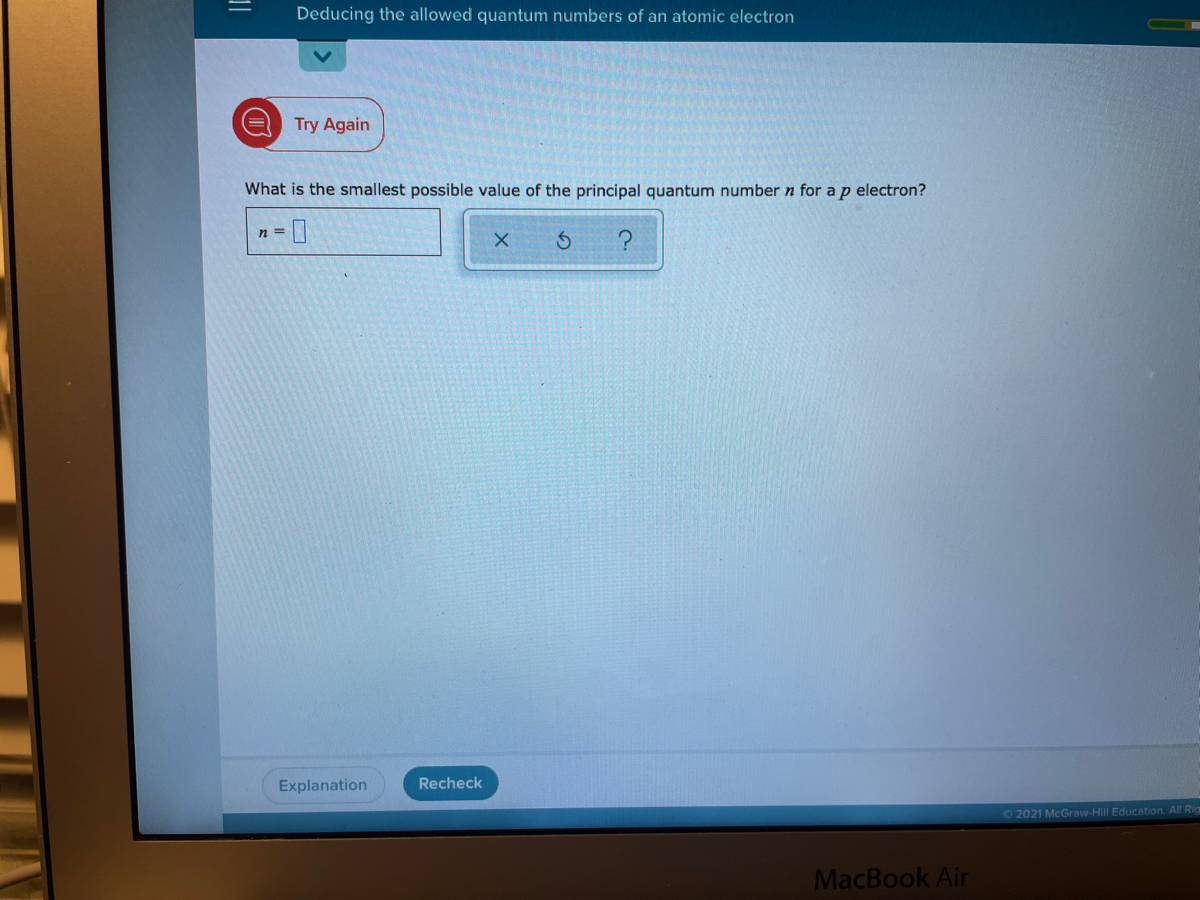
O ELECTRONIC STRUCTURE Deducing the allowed quantum numbers of an atomic electron What is the smallest possible value of the principal quantum number n for an s electron. It covers about 8 cases. Lz ml h 2π ml ll1101l1l L z m l h 2 π m l l l 1 1 0 1.
Principal quantum numbers were in help determine be size an amount of energy of an orbital. List all possible values of the magnetic quantum number m for a 3p. List full potential values of the magnetic quantum enumerate m control a 3p.
It indicates the main energy level occupied by the. View ALEKS- Deducing Quantum Numbers from CHEM 1000 at California State University Bakersfield. List all possible values of the magnetic quantum number m for a 3p electron O ELECTRONIC STRUCTURE Deducing the allowed quantum numbers of an atomic ele.
The principal quantum number is symbolized by n. N is a positive integer so n could be equal to one two three and so on. The angular quantum number l can be any integer between 0 and n - 1.
As the principal quantum number increases so does the size and the energy off the orbitals. Energy n angular momentum ℓ magnetic moment m ℓ and spin m s. R M l ranges from negative one to positive one.
And whenever we have l equals one. 82 Electron configuration atom055 Deducing n and l from a subshell label atom056 Deciding the relative energy of electron subshells atom057 Drawing a box diagram of the electron configuration of an atom atom021 Deducing the allowed quantum numbers of an atomic electron Page 4. The first quantum number describes the electron shell or energy level of an atom.
09272016 Electronic Structure Deducing the. O ELECTRONIC STRUCTURE Deducing The Allowed Quantum Numbers Of An Atomic Electron An Electron In An Atom Is Known To Be In A State With Magnetic Quantum Number M 2. The principal quantum numbers must be whole numbers or intruders.
List full potential values of the magnetic quantum enumerate m control a 3p electron O ELECTRONIC STRUCTURE Deducing the undisputed quantum total of an minute ele. The three quantum numbers n l and m that describe an orbital are integers. Show transcribed shadow text O ELECTRONIC STRUCTURE Deducing the undisputed quantum total of an minute ele.
9272016 ALEKS Student Name. The P comes from the L Value and we see that every p hasnt l equals one. Lets look at the first quantum number here.
This is called the principal quantum number. Apps MyUNM login m UNM email I-. L 1 l where Lz is the z-component of the angular momentum and ml is the angular momentum projection quantum number.
We can describe those electrons in orbitals using the four quantum numbers. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Atom024 Calculating the capacity of electron subshells.
Rules Governing the Allowed Combinations of Quantum Numbers. So when can be won and could be too and could be three in central. III O ELECTRONIC STRUCTURE Deducing the allowed quantum numbers of an atomic electron List all possible values of the angular momentum quantum number for an electron in the M n3 shell of an atom.
The allowed values of n are therefore 1 2 3 4 and so on. So for l equals two remember that the possible values of the magnetic quantum number range from minus l to l. Chemistry questions and answers.
List all possible value of the angular momentum quantum number l for an electron in the L n2 shell of an atom You can solve this problem by using the restrictions on the quantum numbers that label the quantum state of an electron in an atom Youre told an electron is in the L shell of an atom--that means that. So the possible values are minus two minus one zero one and two. The rule in parentheses for the values of ml is that it can range from l to l in steps of one.
This video shows you how to determine or calculate the maximum number of electrons using allowed quantum numbers n l ml and ms. The principal quantum number n cannot be zero. And for each possible magnetic quantum number we also have that I am asking B minus the speed quota number could be minus 12 or plus 12.
This means that end or energy level is four. The value of n ranges from 1 to the shell containing the outermost electron of that atom. And so the quantum numbers for the four p orbital and equals four l equals one and m L ranges from negative one to positive one.

Quantum Numbers The Easy Way Youtube

Quantum Numbers The Easy Way Youtube

Quantum Numbers The Easy Way Youtube

How To Determine The 4 Quantum Numbers From An Element Or A Valence Electron Youtube
Comments
Post a Comment